Latest Developments in Welding Specifications for Sanitary Process Piping
By Barbara K. Henon, Ph.D. and Angel Brond, Arc Machines, Inc, USA
The food, dairy and pharmaceutical industries around the world are under pressure to assure the safety
of their products, to produce them at a lower cost and to higher quality standards than ever before.
Recent, well-publicised problems with food and dairy safety have raised public awareness and concern
about plant conditions.
The need to control the cost of bringing new therapeutic products to market has led the expanding
biopharmaceutical industry in the USA to place an increasing emphasis on quality standards and
documentation in order to expedite the lengthy approval process for new drugs. The FDA (United
States Food and Drug Administration) requires that the facility in which a new drug is produced must
be designed, constructed, and commissioned so that it meets the criteria for process validation.1
Failure to achieve validation on the first attempt can be very costly to the facility owner, so maintaining
quality from the design phase throughout the construction process is essential

| |
Orbital welding operators installing a stainless steel piping
system in a dairy plant in Arizona, USA. Photo courtesy of
Shambaugh & Son, Inc.
|
|
The capability of orbital welding of making a smooth crevice-free inner weld bead on a repeatable basis
has contributed to huge improvements in process piping technology in all of these industries over the
past decade, and Latin America has played a leadership role in accepting and advancing the use of
this technology. Although the food and dairy industries have been slower to accept orbital welding and
other technological advances, this is changing rapidly. As an example of the global economy, the
largest dairy plant in Asia was installed in 1994 with orbital welding equipment from the United States
and stainless steel processing equipment was imported from Europe to make processed cheese slices
for MacDonalds' hamburgers in India. A dairy in Arizona, USA, was recently installed with
state-of-the-art equipment and orbital welding to the highest industry standards. They found that the
time to reach acceptably low bacterial counts in their piping systems was greatly reduced from
previous similar installations done with manual welding.

| |
Stainless steel piping system installed with orbital welding at a pharmaceutical
installation in the UK. Tank bottoms and piping are sloped for durability. Photo
courtesy of Puretech Process Systems.
|
|
Standards for sanitary process piping
Industry standards are important for assuring consistent quality throughout an industry. In the 1950s,
the dairy industry in the United States recognized the importance of good-quality, fullpenetration welds
for maintaining the cleanability of piping systems in dairy plants. The demands of the semiconductor
industry as well as the bioprocess industry for clean, smooth product contact surfaces have led to
advances in process piping technology and equipment fabrication technology, including the use of
orbital welding.
Changes in industry practices have resulted in new standards being written which incorporate these
advances. The new standards include the ASME Bioprocessing Equipment Standard (BPE-97)2, the
AWS D18.1/DI8.2 Specification for welding of austenitic stainless steel tube and pipe systems in
sanitary (hygienic) applications3, and the ISPE series of Pharmaceutical Engineering Baseline®
Guides4. The Baseline guides are not strictly standards, but rather offer guidelines that assist the end
user to comply with FDA regulations for facilities used in the production of drugs.

| |
Hygienic piping systems must be sloped to allow for drainage. Fluid accumulation represents an
unacceptable bioburden. Here a worker checks the level to achieve the specified slope. A 316L
stainless steel tee has been orbitally welded into the 2 in line. Photo courtesy of Niplan, Brazil.
|
3-A Sanitary Standards
5
3-A Accepted Practices for Permanently Installed Product and Solution
Pipelines and Cleaning Systems Used in Milk and Milk Product Processing Plants specified the use of
300 series stainless steel and set guidelines for welding which in the 1950s was all done manually.
The rules were simple: all welds had to be done by the TIG (GTAW) process, which is the same as
that used by automated orbital welding systems today.
Welds had to be fully penetrated to the ID to prevent the formation of crevices which could entrap
product and lead to contamination. The welding surface had to be cleaned prior to welding, and an inert
gas purge was required on the ID of the tubing during welding to prevent oxidation.
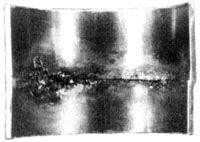 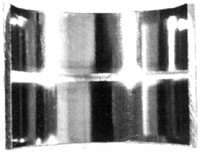
| |
Above Left: Manual weld taken from an operating pharmaceutical plant. Note lack of penetration, misalignment,
discolouration, crevices and protuding material on the product contact surface. This weld would have been
unacceptable by any sanitary piping standard. Above right: orbital weld on 316L stainless steel tubing. Note uniform,
even, fully penetrated weld bead. Weld profile is essentially flat with no concavity or convexity. Parts are well
aligned and the weld has minimal discoloration of the HAZ, meeting most biopharmaceutical specifications.
|
Without a purge, the weld and heat-affected zone (HAZ) on the tube ID would be dark and crusty,
easily corroded, and impossible to maintain in a sanitary condition. There had to be provisions for a
borescope to inspect the inside surface of welds, although no specified number of welds was cited for
inspection. Welds that had crevices, pits, folds, cracks, or other serious defects had to be taken out
and the piping rewelded. 3A also made provisions for pre-production weld samples to be made before
the start of a job and as required during the installation so that installers and owners could reach
agreement on weld quality standards in advance of the job and maintain the agreed upon standards
during the job.
Prior to the publication of the ASME Bioprocessing Equipment Standard in 1997, and the AWS D18.1
standard in 1999 for food and dairy piping systems, the 3-A Standard for permanently installed sanitary
piping system was extensively used by the pharmaceutical food and dairy industries.
FDA, CFR, GMP
The quality inherent in orbital welding joining technology is consistent with the goals
of the CGMP (Current Good Manufacturing Practices) for achieving hygienic food or pharmaceutical
piping systems that will have no adverse affect on the products that pass through them. The FDA will
seek to determine whether newly installed piping systems in these industries meet the requirements of
CGMP. The CGMP Institute is a division of the ISPE (International Society of Pharmaceutical
Engineers).


| |
Industry standards are the key to quality assurance for food, dairy and
pharmaceutical products.
|
The Code of Federal Regulations6
(CFR) Current Good Manufacturing Practice for the Manufacturing,
Packaging, or Holding Human Food, revised in 1989, details the requirements for manufacturing,
preparing and holding of food to prevent its becoming adulterated and unfit for human Consumption.
CFR 21 - §110, Subpart C - Equipment, §110 .40 Equipment and Utensils (a) essentially states that all
equipment and utensils used in food processing must be designed and installed using
corrosion-resistant materials that can be cleaned and maintained in a sanitary condition such that the
condition of the food contact surface will not cause the food to become adulterated. (b) deals with
seams on food contact surfaces which must be smoothly bonded or maintained so as to minimize
accumulation of food particles, dirt, and organic matter and thus minimize the opportunity for growth of
microorganisms.
In validating the critical pharmaceutical process systems in a new or modified facility, the FDA will
establish whether the requirements of three key documents, the CGMP, the ASME (American Society
of Mechanical Engineers) B31.3 Process Piping Code7,
and the project specifications have been met.8
Of these documents, the most significant is 21 CFR §211 (revised November, 1998) which specifies
how the various components in pharmaceutical manufacturing facilities are to be constructed. 21 CFR
§211.65 Subpart D states:
(a) Equipment shall be constructed so that surfaces that contact components, in-process materials, or
drug products shall not be reactive, additive or absorptive so as to alter the safety, identity, strength,
quality, or purity of the drug product beyond the official or other established requirements.
The FDA is very non-specific about how a critical pharmaceutical piping system should be constructed,
but relies upon the requirements detailed in currently accepted industry standards. In the USA, this
would be the ASME B31.3 Process Piping Code and the ASME Bioprocessing Equipment Standard
(BPE-97). It should be noted that a code is required by law, while a standard provides generally
accepted industry practices.
The ASME B31.3 Process Piping Code gives to the Owner ultimate responsibility for documenting to
the FDA that the critical piping systems have been manufactured, fabricated and installed according to
the CGMPs.
ASME Bioprocessing Equipment Standard (BPE-97)
In 1989, representatives of the emerging
bioprocess industry came together with the realization that existing standards did not adequately meet
the need for design and construction of equipment to be used in critical bioprocess piping systems. A
consensus was reached on the need for equipment design that would be both cleanable and
sterilizable. Special emphasis was placed on the quality of weld surfaces once the required strength
was present. The ASME published the ASME Bioprocessing Equipment Standard (BPE-97) in 1997.
Qualification to ASME BPE-97 requires that welds be certified to ASME Section IX of the Boiler and
Pressure Vessel Code9 and ANSI/ASME
B31.3 Process Piping. This requires that a Q.A. manual and
a Q.A. program be in effect with a set of weld standards which reference the BPE Standard. ASME
Section IX is done to verify the structural integrity of the weldments. To meet this requirement sample
welds are subjected to bend tests to verify weld ductility, and tensile testing is done to assure that
welds meet the minimum tensile strength specified for the base material. The results of these tests are
documented as part of the WPS (Weld Procedure Specification) Form QW-482*, and the PQR
(Procedure Qualification Record) Form QW-483*. Welders and welding operators may be qualified by
making acceptable test welds and documenting test results on Form QW-484*. Welder tests require 6
linear inches of weld or multiple coupons, but not more than four samples are required.
While ASME B31.3 was written with manual welding in mind, ASME BPE-97 recommends the use of
orbital or machine welding for bioprocess piping. Manual welding may be used, with the owner's
permission, only when using an orbital weld head would create a deadleg. A deadleg is defined as a
pocket, tee, or extension from a primary piping run that exceeds a defined number of pipe diameters
from the I.D. of the primary pipe. In ASME BPE-97 (Table SD-1) a deadleg (L/D) of 2:1 is considered to
be an achievable target value for bioprocessing systems where L is the length of the extension
measured from the OD of the primary pipe, and D is the I.D. of the extension. Deadlegs are undesirable
because they are difficult to clean and maintain in a sterile condition and may represent an
unacceptable bioburden to the system.
 |
 |
 |
 |
| ASME BPE-97
requires that a minimum of 20% of welds be inspected on the ID
either directly or with a
borescope. |
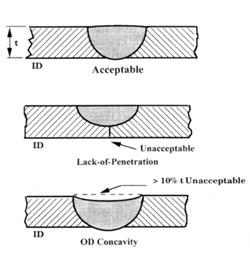
The ASME BPE-97 Standard recognizes the importance of the surface quality of welds for maintaining
the cleanability and sterilizability of piping systems. A smooth internal surface finish of the piping
system, including the welds, is important for controlling the build-up of biofilm that could contaminate
the product. The Materials Joining part of ASME BPE-97 requires that the weld criteria of ASME B31.3
which prohibits weld discontinuities such as cracks, voids, porosity, undercut, lack-of-fusion, and
incomplete penetration that would affect the structural integrity of welds be met but, in addition,
provides visual weld criteria that are important for maintaining the hygienic condition of the piping
system.
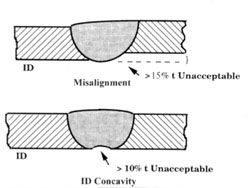
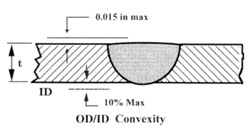
Welds must be fully penetrated with good alignment, with a flat OD and ID profile. An unpenetrated
weld has a crevice which may not be reached by CIP cleaning and becomes a refuge for bacteria.
Excessive I.D. concavity, convexity, or misalignment, that could interfere with proper draining of the
system and allow pooling of fluid where bacteria could gain a foothold and corrosion initiation. An
inspection plan detailing the types of examinations to be made shall be agreed to in advance of the job
by the owner/user and contractor. ASME BPE-97 requires that all welds be inspected visually on the
OD, and that a minimum of 20% be selected at random for internal inspection with a borescope.

| |
Control room of a pharmaceutical plant in the UK designed for packaging a drug for the
treatment of asthma. Process operation is by computer in a tightly-controlled environment.
Process piping was installed with orbital welding. Photo courtesy of Puretech Process Systems.
|
The ISPE Baseline® Guides
The Baseline® series of PHARMACEUTICAL ENGINEERING GUIDES
was developed by ISPE in cooperation with the FDA to establish a baseline approach to new and
renovated facility design, construction commissioning and qualification. The intent is to document
current industry practice for facilities and systems used for production of pharmaceutical products and
medical devices and to avoid unnecessary spending on facility features that have no impact on product
quality.
Vol. 4: Water and Steam Guide is scheduled for publication in November, 2000. Section 12.
Fabrication/Procedures for Distribution Systems discusses material selection for piping systems and
recommends type 316L as the preferred steel for a High Purity Water generation and distribution
system. They give recommendations for limiting the sulphur concentrations of 316L to between 0.005
to 0.020 wt.% to achieve optimal weldability and surface finish. Sections 12.8.2.1.7 and 12.8.2.1.8
include a discussion of passivation of stainless steel after welding to restore the protective surface film
and a description of the different types of rouge, a form of corrosion that may occur in high purity water
distribution systems. Although the protective surface film of stainless steel is disturbed by welding,
studies by Grant et al.10 have shown
that passivation can restore the chrome iron ratio and corrosion
resistance provided an adequate ID purge is provided during welding.
Section 12.8.4.2 Joints using Fusion Butt Welding recommends the use of orbital welding for the
installation of pharmaceutical water systems, citing the smooth inner weld bead. Weld criteria are
presented and suggestions for troubleshooting of welding defects are also provided.
Section 12.8.4.1.2 Clean Preparation Area suggests that a clean room/trailer be used for welding,
bending, and fabrication of High Purity water piping to avoid contamination with metallic or non-metallic
particulates. The use of cleanrooms for orbital welding fabrication has been a standard practice for the
semiconductor industry since the early 1980s. The ISPE Baseline® guide considers the use of
cleanrooms advisable for pharmaceutical installations with similar requirements. It should be pointed
out that a cleanroom, unlike a clean work area, has specific measurable requirements for airborne
particulates regulated by a Federal standard.
| |
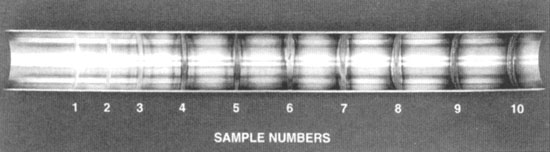
The AWS D18.1/D18.2 Standards for austenitic stainless steel piping in the food and dairy industries have published
a colour photograph showing a series of orbital welds on the ID of a 316L stainless steel tube. The welds were purged
on the ID with argon gas containing oxygen as a contaminant ranging in concentrations from 10 parts per million
(ppm) (sample 1) to 25,000 ppm (sample 10). This provides a basis for owners and contractors to decide upon an
acceptable level for their application. Sample numbers greater than four have typically been considered
unacceptable.
|
AWS D18.1/D18.2
Specification for welding of austenitic stainless steel tube and pipe systems in
sanitary (hygienic) applications. This standard was written by the AWS in cooperation with 3-A to
replace the previous 3-A standard for welding of tubing and pipe in dairy and food product processing
plants. This includes dairy, meat, poultry, vegetable, beverage, and other products consumed by
humans and animals.
Unlike the ASME BPE-97, and the ISPE Baseline® Guides, AWS D18.1 deals exclusively with
welding qualifications and visual examination requirements prior to postweld conditioning. This standard
requires a written Welding Procedure Specification (WPS) with destructive testing of weld samples
according to ANSI/AWS B2.111 and details
acceptance criteria of Procedure Qualification test welds
which must be examined visually and subjected to bend tests and tensile tests according to
ANSI/AWS B4.012 to demonstrate the
strength and ductility of the weldments. Performance
Qualification Variables for welders (manual) and welding operators (orbital) are also detailed. This
standard recognizes manual welding, machine welding and orbital welding and provides guidelines for
weld inspection with maximums given for I.D. and O.D. concavity and convexity, misalignment, and
variation in weld bead width. The results of all weld qualifications and inspections must be
documented/maintained by the contractor and owner.
| |
Orbital welding was used at the Fiocruz Instituto de
Tecnologia em Imunbiologicos vaccine plant near Rio
de Janeiro to install a fermentor for vaccine production
in a class 100,000 cleanroom.
|
Purging the tube or pipe ID
Purging the tube or pipe I.D. during welding is very important for maintaining cleanability during service
of the plant. However, the quality of the I.D. purge always seems to be a negotiable issue since it may
be expensive to provide a completely colour-free weld. Discoloration has been shown to be proportional
to the amount of oxygen (and moisture) in the ID purge gas which is usually argon. Oxygen
concentrations in the low parts per million range in argon will usually produce welds with light or no
discolouration assuming the purge time is sufficient and there are no leaks in the purge system. Both
the D18.1 and D18.2 standards feature a coloured photo showing a series of orbital welds on the ID of
a 316L stainless steel tube. Each weld was purged on the ID with argon gas containing oxygen as a
contaminant at concentrations ranging from 10 parts per million (ppm) (sample 1) to 25,000 ppm
(sample 10).
This provides a basis for owners and contractors to decide upon an acceptance level for their
application. Sample numbers greater than four have typically been considered unacceptable.
Publishing this photo is a big step forward for the industry and the ASME BPE-97 Standard has agreed
to refer to this document for determining acceptance levels for weld discolouration for bioprocess
applications. The ISPE Baseline® guide recommends the use of a cryogenic source (dewar or bulk
gas supply) for purging during welding of high purity pharmaceutical water systems and the use of a
purifier, such as the Nanochem® or GateKeeper™ by Aeronex, which can reduce oxygen and
moisture concentrations to the low parts per billion levels. This level of purge gas purity will usually, but
not always, produce welds with no visible discolouration and better corrosion resistance than that of
more discoloured welds.
Orbital welding SOPs
The reject rates for orbital welding in biopharmaceutical applications have been extremely low. By
refining their standard operating procedures (SOPs) mechanical contractors have documented reject
rates for orbital welding as low as 0.2%. SOPs are written procedures for performing a variety of tasks
so that they are performed in the same way by all personnel in a consistent fashion. This list is not
complete, but would include detailed methods for receiving, handling and storage of materials, tracking
of tubing material heats and control of diameters and wall thicknesses for specific applications.
Proper weld head selection for the tube or pipe being welded, welding procedures, including procedure
for inspection of blind welds, tack-weld procedures, purging procedures such as proper flow rate of inert
gas for each weld head, tungsten type and length determination, fabrication, cutting, end -preparation,
cleaning of weld components, and provision for a clean area set aside for welding would all be included
in the SOPs. This information would be detailed in the project specification prepared by the architect
engineer and the installing contractor and submitted as part of the documentation for validation of the
piping system.
At the outset of a design project by the owner/user and the manufacturer (installing contractor) must
agree on the kind and amount of documentation to be required to present to the FDA at the completion
of the installation. This documentation would typically include weld qualification results consisting of
the WPS, PQR, and WPQ are presented by the installing contractor to the owner/user to present to
the FDA. In addition, the documentation package would include weld maps of bioprocessing
components and weld inspection logs which must include the type of inspection, the date, and welder
identification as well as serial numbers of the orbital welding power supply and weld head.
Material test reports (MTRs) listing the chemical composition, test data of the heats of materials used,
surface finish test reports and results of pressure testing, passivation and other relevant documentation
shall also be retained by the owner/user for a period of at least three years.

| |
Orbital and manual test coupons at a pharmaceutical installation in Brazil. Test coupons by
manual welders indicate the welder's ability to make quality welds. Test coupons done by
orbital welding predict the overall quality of welds in the finished installation.
|
Orbital welding in Latin America
The use of orbital welding is expanding in Latin America for process piping in the pharmaceutical
industry, breweries, food and fruit juice processing, dairies, wineries and cosmetics manufacturing.
Latin American companies have shown considerable interest in bringing their standards in line with the
CGMP. Standards in Latin America are becoming more sophisticated. For example, the Fiocruz
Instituto de Tecnologia em Imunobiologicos used orbital welding in the fabrication of a new building
used for the manufacture of vaccines13. Orbital welding was used for joining the WFI piping, the DI loop
piping, as well as service piping for cold water, air, and steam systems. Type 316L stainless steel
tubing was used for both WFI and DI water systems. Cold water is used for washing glassware used in
the plant, hot DI water is used for rinsing the glassware, and air for drying it. The engineering
contractor, Termo Engen-haria Ltda (TEL) installed a fermentor used in the production of vaccines, in a
class 100,000 cleanroom.
While this is not the same level of classification as cleanrooms typically used in the semiconductor
industry which may be class 100, class 10 or class 1, the same level of technology is used during the
installation and for monitoring particulate levels during operation to assure that it continues to meet the
standard for which it was designed. This level of cleanliness was a precaution to protect the process
from contamination. Other cleanrooms at Fiocruz were used to package the finished product. TEL
wanted to upgrade their welding procedures and standards to a level that would satisfy the FDA in the
USA since Fiocruz intended to export their products. The installation was very successful and Fiocruz
is planning to use orbital welding on another new facility at the same location for the production of
vaccines against viral diseases.
Cosmetics plants in Brazil and Argentina are also upgrading their standards in response to the global
economy. Architect engineers that design facilities and write specifications for pharmaceutical plants
may also design plants for the manufacture of cosmetics and incorporate similar project specifications.
Mechanical contractors may perform work in several industries. A cosmetics plant in Brazil displayed
the weld profile drawings from the ASME BPE-97 standard which they used as a guideline for welds in
a high-purity water system. Pre-production weld samples (test coupons) are used routinely to establish
weld standards in advance of installations and at specified intervals during the course of the job.
A cosmetics plant in Argentina recently specified type 316L sanitary tubing specified to ASTM A 270
with sulphur content limited to between 0.005 and 0.017% similar to the BPE specification with MTRs
to be retained for all tubing and fittings. Provisions were specified for delivery, storage and handling of
materials to maintain cleanliness and all relevant procedures were documented. For example, tubing
and fittings were maintained in protective plastic bags and caps until the time of assembly into the
system.
The gas used for purging on this site had to be sampled before use to assure that it met the required
specification. They used a cryogenic source of argon with trace oxygen less than 2 ppm and moisture
at less than 1.0 ppm. During welding an oxygen analyzer was used to monitor the purge gas leaving
the tubing ID with a maximum acceptable reading of 10,000 ppm prior to welding. While this is much
higher than would be required in a high-purity semiconductor application, it indicates a high level of
awareness of the need for a good purge and demonstrates use of state-of-the-art fabrication technology
to assure that the specified conditions were met.
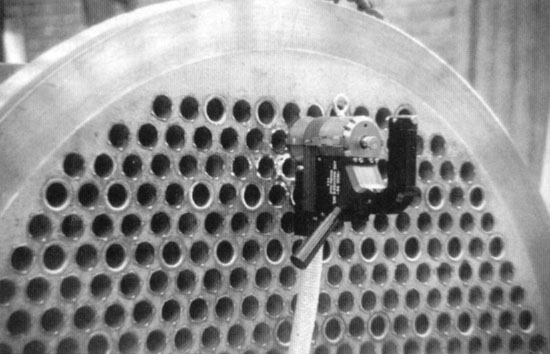
| |
Inoxcol used an Arc Machines Model 96 tube-to-tubesheet weld head to weld this heat exchanger used for the
lyophillization of coffee in Columbia.
|
Tack-welding procedures were also specified. An alignment gauge was specified to hold the weld
components in position for welding and provisions for an I.D. purge during tack-welding were made.
Tack-welding procedures were written to assure that the tacks would have no deleterious effect on the
finished orbital welds. The same company specified that only trained operators be allowed to operate
the orbital welding equipment. Training for orbital welders included welding under field conditions to
prepare the operators for working under adverse conditions. These specifications for the manufacture of
cosmetic products were as detailed and comprehensive as those for modem bioprocessing plants.
Heat exchangers. Sanitary process piping may sometimes include heat exchangers. Inoxcol in
Columbia recently used an Arc Machines Model 96 tube-to-tubesheet weld head for the orbital welding
of 316L tubing to tubesheets in heat exchangers used for the Iyophillization of coffee in the production
of instant (powdered) coffee. Other heat exchanger applications such as using 316L stainless steel for
a reverse osmosis system are in the planning stages.
Productivity. A number of breweries in Mexico and Latin America have begun to use orbital welding,
not only for repeatability of weld quality, but because orbital welding can result in demonstrably higher
productivity than can be achieved with manual welding. The "Quilmes"14 brewery in Argentina recently
completed a project using an AMI Model 96-6625 weld head which has the capability of adding filler
metal to the weld.
Productivity gains were achieved through reduced time to weld each joint as well as lower reject rates
and reduced need for rework. On this application, a night crew prepared assemblies for welding by
cutting, prepping and tack welding elbows and reducers to opposite ends of a tube and setting up the
purge. The single orbital welding operator was able to complete over 60 sanitary-quality welds a day
which was three times faster than manual welding on the same application with no rejections. Thus
orbital welding is eminently suitable for "fast-track" construction projects without sacrificing quality.
Summary
Increasing concern for the safety and integrity of food, dairy and pharmaceutical products and the need
to bring biopharmaceutical products to market in a timely fashion has led to improvements in industry
standards to facilitate the cleanability and sterilizability of product contact surfaces. The
biopharmaceutical industry recognizes that the orbital welding process makes it possible to
consistently achieve crevice-free welds with a smooth surface which decreases the affinity for
colonization and growth of microorganisms and increases the efficacy of CIP. Orbitally welded
systems, installed with proper fabrication techniques and documentation, are in compliance with 21
CFR §211 Subpart D facilitating piping system validation as well as providing excellent performance in
service. This has made orbital welding the preferred joining technology for the biopharmaceutical
industry in the United States.
The cost of orbital welding and other advances in fabrication technology must be considered in light of
the high cost of contamination of food and pharmaceutical products which may be distributed to a
worldwide community.
The use of orbital welding is expanding in Latin America. Cosmetics plants, breweries, and food and
dairy processing plants are upgrading their standards and using technology previously limited to the so
called high-purity industries.
References
- FDA Code of Federal Regulations: Current Good Manufacturing Practice for the Manufacture,
Processing, Packaging, or Holding of Drugs. 21 CFR - Parts 210 & 211, November 4, 1998. GMP
Institute, 6899 Steger Drive, Cincinnati, OH 45237 Tel: +1 (513) 697-2666.
- ASME Bioprocessing Equipment Standard (BPE-97). ASME, 22 Law Drive, Box 2900, Fairfield, New
Jersey 07007-2900.*
- AWS D18.1:1999. Specification for welding of austenitic stainless steel tube and pipe systems in
sanitary (hygienic) applications. American Welding Society, 550 N.W. LeJeune Rd. Miami, Florida
Tel:+ 1-305/443-9353*
- ISPE Baseline® Pharmaceutical Engineering Guides Vol. 4. Water and Steam Guide. Draft Working
Document (Revision B) Revised October 30, 1997. ISPE, 3816 W. Linebaugh Ave., Suite 412, Tampa,
FL 33624, USA Tel: +1-813/960-2105 Fax: +1-813/264-2816.
- 3-A Accepted Practices for Permanently Installed Product and Solution Pipelines and Cleaning
System Used in Milk and Milk Product Processing Plants. 3-A Sanitary Standards, 1451 Dolley
Madison Blvd., McLean, VA 22101-3850.
- FDA Code of Federal Regulations: Current Good Manufacturing Practice for the Manufacture,
Processing, Packaging, or Holding of Human Food. 21 CFR - Parts 110, September, 1989. GMP
Institute, 6899 Steger Drive, Cincinnati, OH 45237 Tel: +1 (513) 697-2666.
- ASME B31.3 Process Piping Code (1996 Edition) ASME, 22 Law Drive, Box 2900, Fairfield, New
Jersey 07007-2900.*
- Lohnes, J. Codes and standards: Critical process piping systems requirements as keys to success.
ISPE San Diego Chapter Newsletter, Issue #4, 1999.
- ASME Section IX of the Boiler and Pressure Vessel Code. ASME, 22 Law Drive, Box 2900, Fairfield,
New Jersey 07007-2900.
- Grant, A., B.K. Henon, and F. Mansfeld. Effects of purge gas purity and chelant passivation on the
corrosion resistance of orbitally welded 316L stainless steel tubing. Pharmaceutical Engineering,
January/February, March/April, 1997.
- ANSI/AWS B2.1, Specification for Welding Procedure and Performance Qualification. American
Welding Society, 550 N.W. Lejeune Rd. Miami, Florida Tel: + 1-305/443-9353.
- ANSI/AWS B4.0, Standard Methods for Mechanical Testing of Welds. American Welding Society,
550 N.W. Lejeune Rd. Miami, Florida. Tel: +1-305/443-9353.
- Henon, B.K. and Angel Brond. Orbital TIG provides right connections for vaccine plant. Welding and
Metal Fabrication, April, 1998.
- Henon, B.K. and Angel Brond. Orbital welding technology for pharmaceutical piping systems. Tube
International, June,2000.
- Henon, B.K. Orbital welding in compliance with the new ASME Bioprocessing Equipment Standard
(ASME BPE-97). Pharmaceutical Engineering, January/February, 1999.
* Many of these standards are available on the Internet:
For ASME, go to www.asme.org. Click on "Publications". For "Keyword" put in Bioprocessing
Equipment, "Search By" Codes and Standards Designator and then click Search. That will take you to
the standard and you can then put it in your shopping cart. After that, return to Publications and put in
BVPOC Section 9 and then B31.3 (see following page).
For AWS, go to www.aws.org. Click on "Technical" on the homepage. In Technical, click on AWS
Technical Committees and Standards Program. On that page, click on D18.
For ASTM, go to www.astm.org. Click on "ASTM" Store: then "Search for Individual Standards." In
"Search For:" put in A269 or A270. These can be delivered online if you have Adobe Acrobat.
All of these organizations allow purchasing online by credit card.
This paper was first presented at the Segundo Simposium Internacional de Soldadura SIS2000, ITESM
Campus Monterrey, Nuevo Leon, Mexico (August 29-31, 2000)
By Barbara K. Henon, Ph.D. and Angel Brond, Arc Machines, Inc, USA
Arc Machines, Inc
- Addr: 10500 Orbital Way, Pacoirna, CA 91331, CA, USA
- Tel: 1-818-896-9556
- Fax: 1-818-890-3724
- WWW: http://www.arcmachines.com/
- Email:
- Contact: Pauline Kim
|